Scientists Discover Why Promising Alzheimer's Drugs Keep Failing, and Point to a Fix
Discovery could redirect drug development for disease affecting 6.7 million Americans
The two AMPKα isoforms can have opposing effects on cognitive function. This is critical for understanding why some drugs helped while others made patients worse.”
WINSTON-SALEM, NC, UNITED STATES, January 7, 2026 /EINPresswire.com/ -- A comprehensive analysis published in the journal Brain Medicine (Genomic Press, New York) may finally explain one of the most frustrating puzzles in Alzheimer's research: why drugs targeting a key cellular energy sensor have produced wildly inconsistent results, sometimes helping patients and sometimes making them worse.— Tao Ma, PhD, Professor, Wake Forest University School of Medicine
The answer, according to researchers at Wake Forest University School of Medicine, lies in a distinction that scientists have largely overlooked for years. The enzyme known as AMPK, which acts as an energy thermostat in every cell, actually exists in two different forms, and these twin versions appear to have opposing effects on memory and brain function.
"For years, the field has treated AMPK as a single entity when investigating its role in Alzheimer's disease," said Dr. Tao Ma, Professor at Wake Forest University School of Medicine and senior author of the study. "This distinction is critical for understanding why some pharmacological approaches have shown benefit while others have worsened outcomes."
AMPK sits at the crossroads of cellular energy management and memory formation. Neurons are the most energy-hungry cells in the human body, and the enzyme helps them balance their metabolic books while also controlling the production of proteins essential for forming lasting memories. When Alzheimer's disease develops, both of these processes go awry.
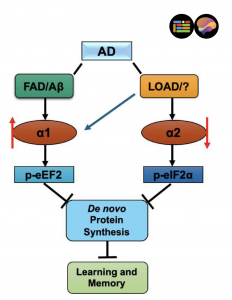
The two forms of AMPK's catalytic subunit, called α1 and α2, share 90 percent of their structure in the regions that perform their main work. But the Wake Forest team's synthesis of recent research reveals that this small difference translates into dramatically different roles in the brain.
Examination of brain tissue from deceased Alzheimer's patients showed a striking pattern: the α1 form was significantly elevated while the α2 form was significantly reduced. Notably, this imbalance did not appear in patients with other types of dementia, including Lewy body dementia and frontotemporal dementia, suggesting it represents a signature specific to Alzheimer's disease.
Studies in mouse models of Alzheimer's disease demonstrated that suppressing the α1 form restored learning and memory deficits, while suppressing the α2 form had no such benefit. These improvements occurred regardless of whether the mice still showed the characteristic amyloid plaques associated with the disease.
The findings may help resolve the controversy surrounding metformin, a widely prescribed diabetes medication that activates AMPK. Some studies have suggested metformin protects against Alzheimer's, while others have found it may increase cognitive problems. The new analysis suggests that metformin may activate different AMPK forms in different cell types and brain regions, potentially explaining the contradictory findings.
The review identifies several promising directions for future treatment development, including the creation of drugs that can cross into the brain and selectively target one AMPK form while leaving the other alone. Early work has already shown that certain compounds preferentially interact with specific AMPK forms, raising hope that more precise therapies could succeed where broad-spectrum approaches have failed.
A pilot study found that blood levels of the α1 form were significantly lower in patients diagnosed with Alzheimer's disease and mild cognitive impairment compared to healthy individuals, suggesting these measurements might eventually contribute to diagnostic testing.
Alzheimer's disease currently affects an estimated 6.7 million Americans and remains the only condition among the top ten causes of death without a treatment that can cure or substantially slow its progression.
The research was supported by the National Institutes of Health and the Cure Alzheimer's Fund. Co-authors include Helena R. Zimmermann and Hannah M. Jester of Wake Forest University School of Medicine, and Dr. Robert Vassar of Northwestern University Feinberg School of Medicine.
The peer-reviewed mini-review article, "Isoform-specific roles and overlooked complexity of AMPKα in Alzheimer's disease," is freely available via Open Access in Brain Medicine at: https://doi.org/10.61373/bm026y.0003
About Brain Medicine: Brain Medicine (ISSN: 2997-2639, online and 2997-2647, print) is a peer-reviewed medical research journal published by Genomic Press, New York. The journal covers the underlying science, causes, outcomes, treatments, and societal impact of brain disorders across all clinical disciplines.
Source-Genomic Press: https://genomicpress.com/
Ma-Li Wong
Genomic Press
mali.wong@genomicpress.com
Visit us on social media:
X
LinkedIn
Bluesky
Instagram
Facebook
Legal Disclaimer:
EIN Presswire provides this news content "as is" without warranty of any kind. We do not accept any responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you have any complaints or copyright issues related to this article, kindly contact the author above.